alternative splicing
Alternative splicing (AS) is a closely regulated, variable adaptation of the routine RNA modification process of pre-mRNA splicing. Alternative splicing is a form of epigenetic mechanism that enables a single gene to give rise to multiple, differentially spliced versions of a protein, increasing complexity without a change in the genome.
In 1980, a gene called IgM provided the first recognized example of alternative splicing in cells—there were earlier examples in viruses. It has since been demonstrated that cells employ alternative splicing to increase protein diversity toward a variety of biological ends.
An average mammalian gene possesses eight or nine exons—since most human genes undergoing some form of alternative splicing, virtually all of these exons are candidates for elaborate control. The human genome contains 3164.7 million nucleotide bases and only around 25,000 genes, much fewer than the 90,000 proteins that we are estimated to manufacture, and much fewer than prior estimates of 80,000-140,000 expressed sequence tags (ESTs). Human Genome Project
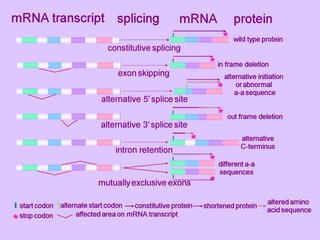 A cell typically splices a single mRNA transcript in multiple ways to generate an assortment of proteins. Alternatively spliced introns tend to lie between those exonal segments of a gene that encode the functional units, or domains, of a protein (ORF).
A cell typically splices a single mRNA transcript in multiple ways to generate an assortment of proteins. Alternatively spliced introns tend to lie between those exonal segments of a gene that encode the functional units, or domains, of a protein (ORF).
Alternative splicing can alter the mRNA product in several ways. (click to enlarge image at left)
At the simplest level, an exon can be removed (exon skip), lengthened or shortened (alternative 5'AS or 3'AS splicing). Thus, observed mechanisms of alternative splicing alteration include exon skipping, intron retention, and the use of an alternative splice donor or acceptor site. [r]
Alternative splicing can affect alternative promoters, internal exons, or alternative terminal exons, and generates segments of mRNA variability that shift the reading frame, insert or remove amino acids, or introduce a premature termination codon (Fig. 2). The variable segment within the mRNA results from insertion/deletion, or a mutually exclusive swap. The effects on coding potential are an in-frame insertion or deletion, a reading-frame shift, or introduction of a stop codon. mRNAs containing a stop codon >50 nt upstream of the position of the terminal intron are degraded by nonsense-mediated decay. Therefore, introduction of a premature termination codon into an mRNA by alternative splicing can be a mechanism to down-regulate expression of a gene. Alternative splicing also affects gene expression by removing or inserting regulatory elements controlling mRNA stability (NMD or nonstop decay), translation, or localization.
Exon skipping is the most frequent alternative splicing mechanism known in mammals, constituting about 40% of AS events, and as such is a major contributor to mammalian protein diversity. Exon skipping results in the loss of an exon in the alternatively spliced mRNA.
Alternative donor 5'AS or alternative acceptor 3'AS splicing together contribute to 25% of all AS events conserved between humans and mice (4).
Intron retention is defined by the presence of a transcript-confirmed intron within a transcript-confirmed exon. Intron retention occurs when introns are not spliced out of the RNA transcript, resulting in the intron(s) being retained within the mRNA as part of an exon. This lengthening mechanism the commonest form of alternative splicing in plants and lower multicellular organisms.
Regulation of AS:
Alternative splicing depends upon a splice-site and nearby enhancer and repressor sequences—short segments of RNA that couple with regulatory proteins. It has been estimated that the splicing of a single exon may be promoted by at least three to seven enhancer sequences.
As a result of alternate splicing, mutations that alter a splice site or a nearby regulatory sequence can have subtle effects by shifting the ratio of the resulting proteins without entirely eliminating any form.
Regulation of alternative splicing is often temporal or tissue-specific, generating different protein isoforms in different developmental states or different tissues. At the level of the organism, specific isoforms are known to be produced as a consequence of regulation by extracellular signaling mechanisms. Alternative splicing can allow one gene to generate different proteins in different tissues, providing the diversity encoded within genomes that are smaller than would be expected from gene expression by the organism. Many highly specialized brain proteins arise from differential splicing of genes that are also expressed in other tissues. Cells can even modify splicing in response to changing conditions, and not only can alternative splicing tweak the structure of a single protein, but it may also be a means of regulating entire pathways.
Recent bioinformatics studies have demonstrated that AS-prone exons can be distinguished from constitutively spliced exons by several features(1).
1. Divisibility by three of the exon length, which is likely to ensure the preservation of the reading frame in the mRNA (3).
2. Evolutionary conservation of intronic sequences flanking AS exons (3–8). Though not yet fully understood, the unusually high conservation of the introns that surround AS exons suggests the presence of cis-regulatory elements with regulatory proteins (5).
Alternative splicing is controlled by the binding of trans-acting protein factors to cis-acting sequences within the pre-mRNA, which result in differential use of splice sites. Since the splice sites do not contain enough information to explain the complex regulation of AS, this fine-tuned mechanism is achieved by multiple weak signals across the exons and introns which are recognized by an extensive number of different proteins (2). Many such alternative-binding sequences have been identified and are grouped as either enhancer or suppressor elements. These elements are typically short (8-10 nucleotides) and are less conserved than the splice sites at exon-intron junctions. The elements are either intronic or exonic, and either enhancers or silencers depending upon whether they increase or decrease splicing – ISE, ISS, ESE, ESS.
Members of the SR (serine rich) splicing factors control alternative splice site recognition by binding to the splicing enhancer and inhibitor elements (ESE, ESS, ISE, ISS). The SR proteins interact with the pre-mRNA substrate and with snRNP proteins (small nuclear ribonucleoproteins , pronounced 'snurps'). The role of SR splicing factors in regulating splice site selection is believed to occur in arginine-serine (RS) domain dependent and RS domain independent mechanisms.
animation of alternative splicing if this link does not work then click on figure 1 of alternative splicing link : life cycle of an mRNA ~ click on Quicktime Q :HHMI Feature Article on Alternative Splicing : Artist's conception of ASControlling the Synapse — 49 Proteins at a Time : The Alternative Splicing Website : Alternative Splicing DB (ASDB) : DNA-RNA-ProteinNational Center for Biotechnology Information
In 1980, a gene called IgM provided the first recognized example of alternative splicing in cells—there were earlier examples in viruses. It has since been demonstrated that cells employ alternative splicing to increase protein diversity toward a variety of biological ends.
An average mammalian gene possesses eight or nine exons—since most human genes undergoing some form of alternative splicing, virtually all of these exons are candidates for elaborate control. The human genome contains 3164.7 million nucleotide bases and only around 25,000 genes, much fewer than the 90,000 proteins that we are estimated to manufacture, and much fewer than prior estimates of 80,000-140,000 expressed sequence tags (ESTs). Human Genome Project
 A cell typically splices a single mRNA transcript in multiple ways to generate an assortment of proteins. Alternatively spliced introns tend to lie between those exonal segments of a gene that encode the functional units, or domains, of a protein (ORF).
A cell typically splices a single mRNA transcript in multiple ways to generate an assortment of proteins. Alternatively spliced introns tend to lie between those exonal segments of a gene that encode the functional units, or domains, of a protein (ORF).Alternative splicing can alter the mRNA product in several ways. (click to enlarge image at left)
At the simplest level, an exon can be removed (exon skip), lengthened or shortened (alternative 5'AS or 3'AS splicing). Thus, observed mechanisms of alternative splicing alteration include exon skipping, intron retention, and the use of an alternative splice donor or acceptor site. [r]
Alternative splicing can affect alternative promoters, internal exons, or alternative terminal exons, and generates segments of mRNA variability that shift the reading frame, insert or remove amino acids, or introduce a premature termination codon (Fig. 2). The variable segment within the mRNA results from insertion/deletion, or a mutually exclusive swap. The effects on coding potential are an in-frame insertion or deletion, a reading-frame shift, or introduction of a stop codon. mRNAs containing a stop codon >50 nt upstream of the position of the terminal intron are degraded by nonsense-mediated decay. Therefore, introduction of a premature termination codon into an mRNA by alternative splicing can be a mechanism to down-regulate expression of a gene. Alternative splicing also affects gene expression by removing or inserting regulatory elements controlling mRNA stability (NMD or nonstop decay), translation, or localization.
Exon skipping is the most frequent alternative splicing mechanism known in mammals, constituting about 40% of AS events, and as such is a major contributor to mammalian protein diversity. Exon skipping results in the loss of an exon in the alternatively spliced mRNA.
Alternative donor 5'AS or alternative acceptor 3'AS splicing together contribute to 25% of all AS events conserved between humans and mice (4).
Intron retention is defined by the presence of a transcript-confirmed intron within a transcript-confirmed exon. Intron retention occurs when introns are not spliced out of the RNA transcript, resulting in the intron(s) being retained within the mRNA as part of an exon. This lengthening mechanism the commonest form of alternative splicing in plants and lower multicellular organisms.
Regulation of AS:
Alternative splicing depends upon a splice-site and nearby enhancer and repressor sequences—short segments of RNA that couple with regulatory proteins. It has been estimated that the splicing of a single exon may be promoted by at least three to seven enhancer sequences.
As a result of alternate splicing, mutations that alter a splice site or a nearby regulatory sequence can have subtle effects by shifting the ratio of the resulting proteins without entirely eliminating any form.
Regulation of alternative splicing is often temporal or tissue-specific, generating different protein isoforms in different developmental states or different tissues. At the level of the organism, specific isoforms are known to be produced as a consequence of regulation by extracellular signaling mechanisms. Alternative splicing can allow one gene to generate different proteins in different tissues, providing the diversity encoded within genomes that are smaller than would be expected from gene expression by the organism. Many highly specialized brain proteins arise from differential splicing of genes that are also expressed in other tissues. Cells can even modify splicing in response to changing conditions, and not only can alternative splicing tweak the structure of a single protein, but it may also be a means of regulating entire pathways.
Recent bioinformatics studies have demonstrated that AS-prone exons can be distinguished from constitutively spliced exons by several features(1).
1. Divisibility by three of the exon length, which is likely to ensure the preservation of the reading frame in the mRNA (3).
2. Evolutionary conservation of intronic sequences flanking AS exons (3–8). Though not yet fully understood, the unusually high conservation of the introns that surround AS exons suggests the presence of cis-regulatory elements with regulatory proteins (5).
Alternative splicing is controlled by the binding of trans-acting protein factors to cis-acting sequences within the pre-mRNA, which result in differential use of splice sites. Since the splice sites do not contain enough information to explain the complex regulation of AS, this fine-tuned mechanism is achieved by multiple weak signals across the exons and introns which are recognized by an extensive number of different proteins (2). Many such alternative-binding sequences have been identified and are grouped as either enhancer or suppressor elements. These elements are typically short (8-10 nucleotides) and are less conserved than the splice sites at exon-intron junctions. The elements are either intronic or exonic, and either enhancers or silencers depending upon whether they increase or decrease splicing – ISE, ISS, ESE, ESS.
Members of the SR (serine rich) splicing factors control alternative splice site recognition by binding to the splicing enhancer and inhibitor elements (ESE, ESS, ISE, ISS). The SR proteins interact with the pre-mRNA substrate and with snRNP proteins (small nuclear ribonucleoproteins , pronounced 'snurps'). The role of SR splicing factors in regulating splice site selection is believed to occur in arginine-serine (RS) domain dependent and RS domain independent mechanisms.
animation of alternative splicing if this link does not work then click on figure 1 of alternative splicing link : life cycle of an mRNA ~ click on Quicktime Q :HHMI Feature Article on Alternative Splicing : Artist's conception of ASControlling the Synapse — 49 Proteins at a Time : The Alternative Splicing Website : Alternative Splicing DB (ASDB) : DNA-RNA-ProteinNational Center for Biotechnology Information










































0 Comments:
Post a Comment
<< Home